Sources: The best source for RGB filters is rainbowsymphony They sell a wonderful Color Paddles Set that includes props we will use for other scenes and that also shows the spectral transmission charts for the RGB gels. They also sell a set of six 8 by 10 inch colored filters that comes with a diffraction grating sheet for $19.95. The six colored gels in your SWAG come from these 8 by 10 inch sheets. As usual Amazon.com is also a source and some of the rainbowsymphony products are available through them.
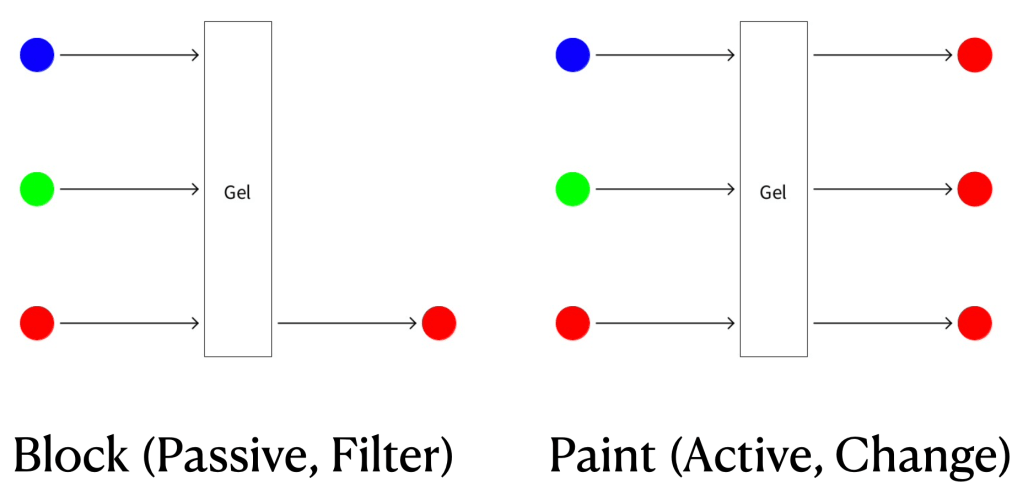
Two Models: Your students (and you) should come up with two plausible models. The first model, shown on the left below, is a “passive,” “filter” or “block” model, which says that each gel lets some colors through unchanged but blocks others. Thus, a red gel lets red through but blocks green and blue. The second model, shown on the right below, is an “active,” “change” or “paint” model, which says that the gel lets some colors through unchanged but transforms or “paints” others. Thus, a red gel lets red through unchanged but paints green and blue light red.
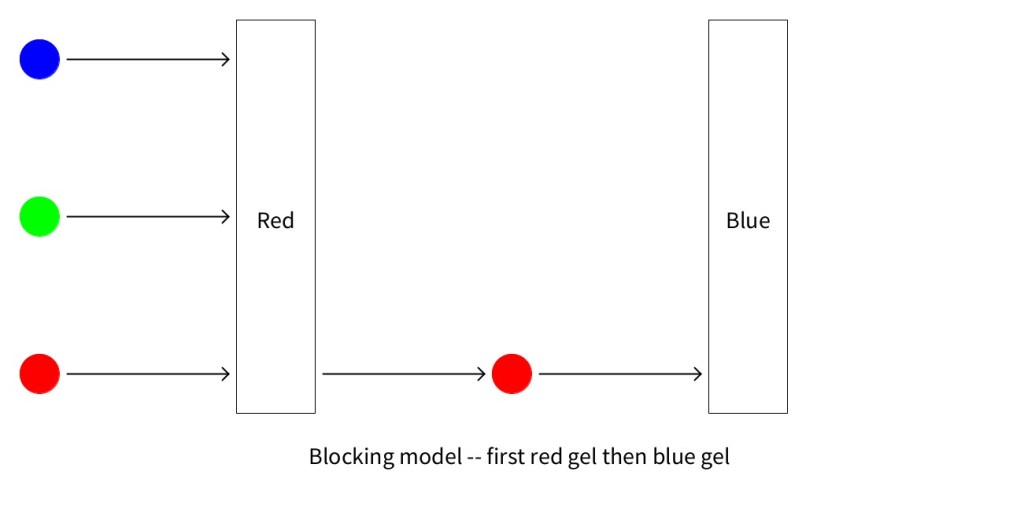
The two figures below show the predictions made by the block model for a sandwich of a red and blue model. Notice that this model predicts that sandwich of red and blue lets no light through and it doesn’t make any difference whether light passes through the red gel first or the blue gel first.

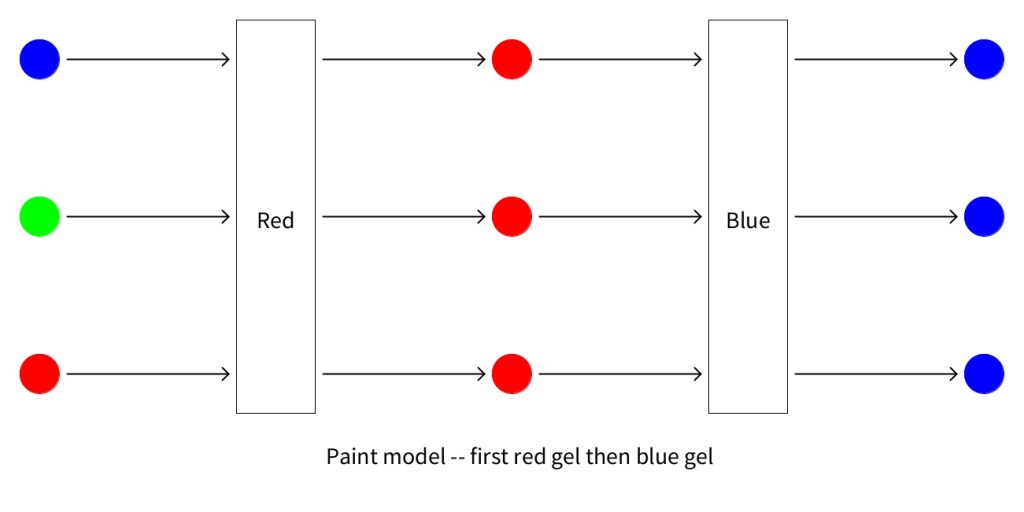
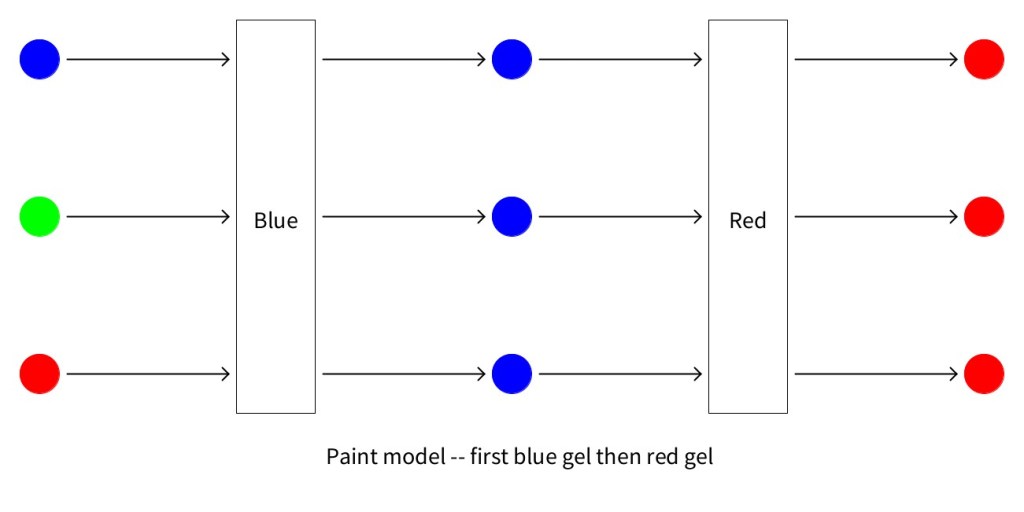
The two figures below show the predictions made by the paint model for a sandwich of a red and blue model. Notice that it does make a difference whether light passes through the red gel first or the blue gel first.
The experiment below supports the passive, block model and rejects the active, paint model.

Another interesting experiment supporting the passive, block model is shown below.

The passive, block model does a great job explaining these gels but it fails in other situations. You can do an interesting experiment using chlorophyll from leaves to see why more active models are needed.
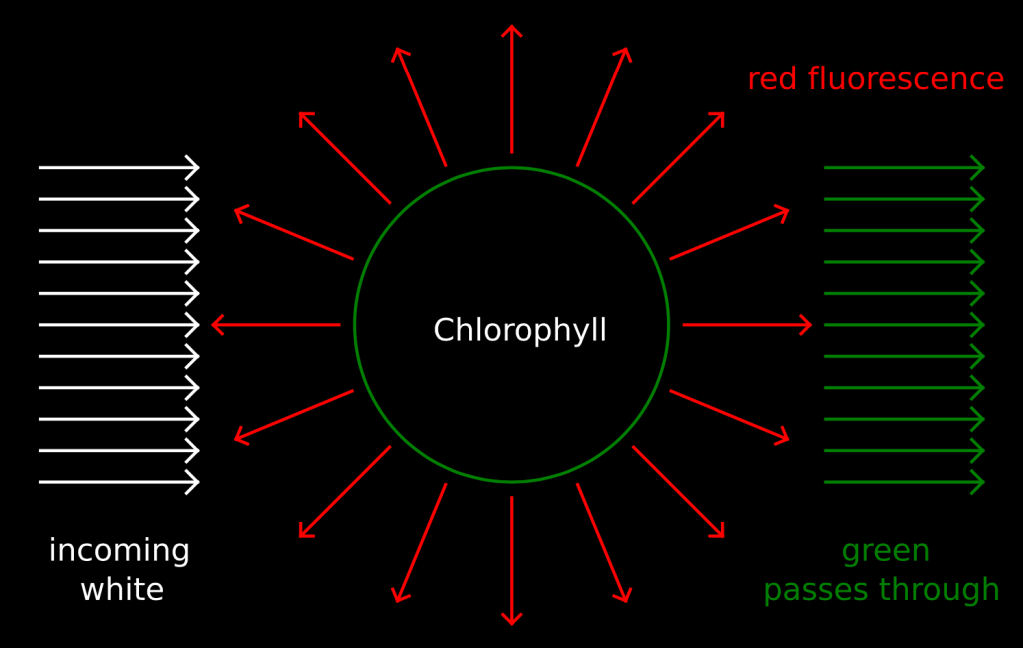
When white light interacts with a chlorophyll molecule green passes through but some of the other colors do not. The energy from light that is not passed through is used in various ways. Some of this energy interacts with atoms in the chlorophyll molecule and kicks electrons in those atoms up to higher and unstable levels. When these electrons fall back down they emit red light in all directions. This is called “fluorescence.” Click here for more information on how to do this experiment and more about the mechanisms behind the interactions between light and chlorophyll. Our observations in this experiment lead us to a deeper understanding of chemistry.